H2O2 VHP PASS BOX
Pass box is a kind of auxiliary equipment for clean room, mainly used for the transfer of small items between clean area and clean area, clean area and non-clean area, in order to reduce the number of clean room opening, reduce the pollution of clean room to the minimum degree. The passbox is made of stainless steel plate, which is smooth and smooth. Double door interlocking, effectively prevent cross contamination, with electronic or mechanical interlocking device, and equipped with ultraviolet sterilization lamp.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
1. Main uses

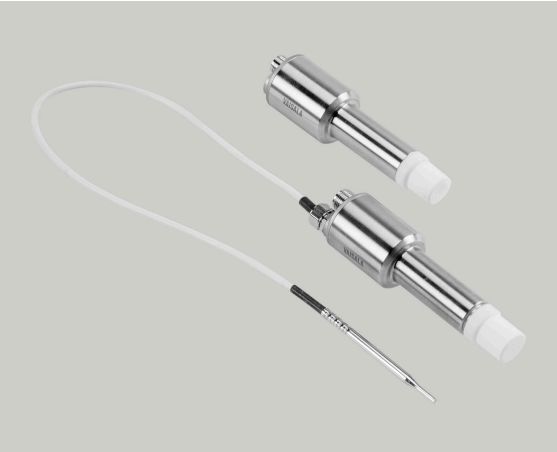
Hydrogen peroxide (VHP) sterilization pass box by integrating vaporization of hydrogen peroxide sterilization sterilization on all items exposed surfaces inside the pass box, using hydrogen peroxide gas than the liquid state at room temperature is more advantage of the ability to kill spores, the generation of free hydroxyl, ingredients used to attack cells, including lipid, protein and DNA, instead of traditional ultraviolet disinfection method, to achieve a total sterilization requirements; Vaporized hydrogen peroxide sterilization is suitable for low-temperature sterilization of products. Vaporized hydrogen peroxide has good material compatibility, easy to decompose, and eventually decompose into water and oxygen, without harmful residue. Mainly used in pharmaceutical industry aseptic clean room and clean room between the material transfer, can achieve the requirements of aseptic material transfer.
2. Performance characteristics
- The whole line design, material, manufacture, assembly, debugging and so on met the new GMP pharmaceutical production specification requirements;
- Using hydrogen peroxide vapor (VHP) as biological detergent, it can effectively kill fungi, bacteria, spores and viruses;
- VHP is the low temperature, atmospheric pressure condition of decontamination technology, broad spectrum, high efficiency, environmental protection; Hydrogen peroxide steam sterilization is also the United States, China and other countries in the pharmacopoeia "sterilization method" included in the method;
- VHP pass box uses the elevation arc structure, modular design, on-site assembly;
- In biological decontamination and exhaust phase, all the air into the chamber through the H14 HEPA filter, prevent secondary pollution materials;
- Room temperature sterilization cycle time: < 120 min (according to the biological material load, load method, and validation method);
- VHP residue levels: < 1 PPM (residue level determined by the technological requirements);
- Sterilization concentration curve range: 0-1000 ppm;
- Chamber sealing effect: in 500 pa pressure, leakage rate is not more than 5% within 10 minutes
- Wind speed: 0.35 to 0.65 m/s, the wind speed is adjustable;
- Has many alarm functions: high pressure and low pressure alarm; Opening overtime alarm; Lock the door and alarm
- The VHP pass box has sterilization cycle parameters data saving and printing functions;
- The VHP pass box has high efficient filtered air flow protection, when open both doors, it will form an airlock status to prevent cross contamination;
- Inlet and discharge to the 2 doors structure, with a pneumatic sealing, locking and the working status of the two-door interlock function, reliable sealing;
- Can achieve fast and efficient sterilization, equipment operation with low cost and easy for verification;
- Has exhaust device, remove excess hydrogen peroxide decomposition, avoid residues
- Automatic hydrogen peroxide charging device can be configured, add fluid can be automatically according to the sterilization conditions;
- Configurable high and low concentration detection system, detection of hydrogen peroxide concentration in the process of sterilization;
- Configurable cavity internal cart type sterilization bracket and rail system, supporting facilities, such as cavity in vitro transfer car, convenient material handling;
- Configurable data acquisition system, can hold, such as temperature, humidity, vacuum pressure data in real-time detection and real-time print;
- Can be configured to monitor hydrogen peroxide concentration of gas, dust particles and planktonic bacteria port;
- Configurable integrated VHP generator system without external VHP generator, better evaporated into gas, the hydrogen peroxide liquid to achieve better effect of sterilization, sterilization programmed temperature, accused of wet
- Configurable chamber leakage rate automatic test procedures; With leak alarm function;
- Adopts programmable controller (PLC) automation control equipment at various stages of operation, with the human touch screen operation interface, operation process clear, system work stable and reliable;
- Control convenient and good sterilization effect, energy saving and high security;
- Can realize customization according to customer's actual demand.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
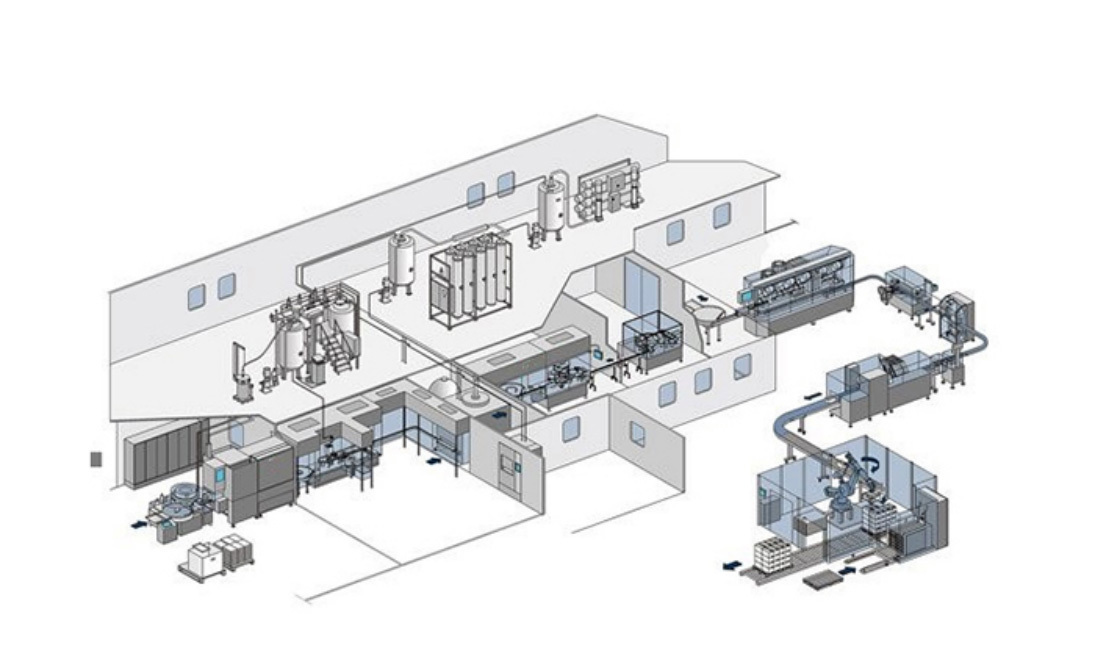
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
H2O2 VPH PASS BOX
pass box
VHP pass box
Previous:
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131
































