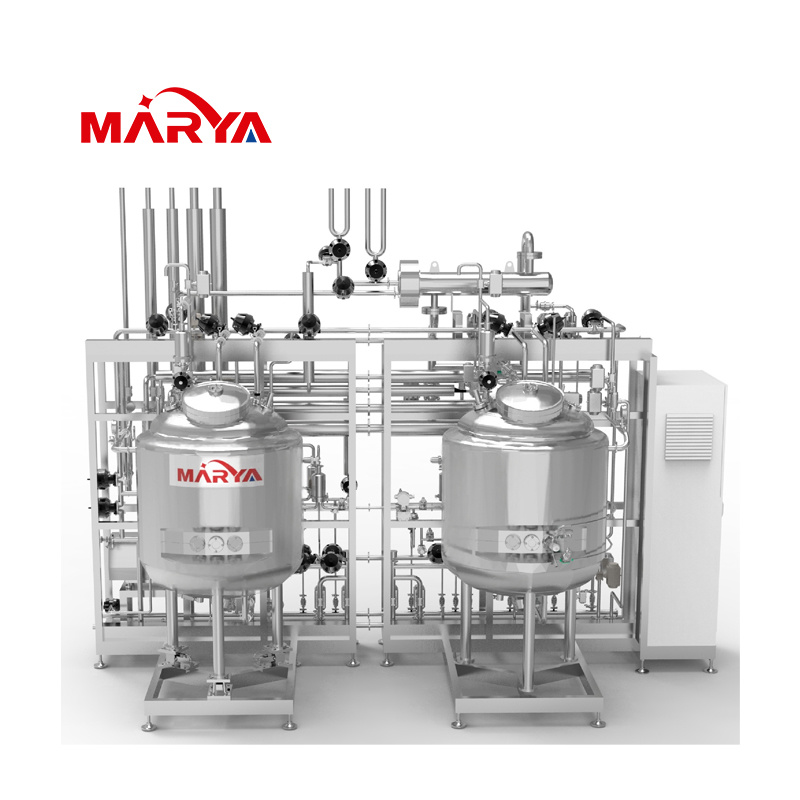
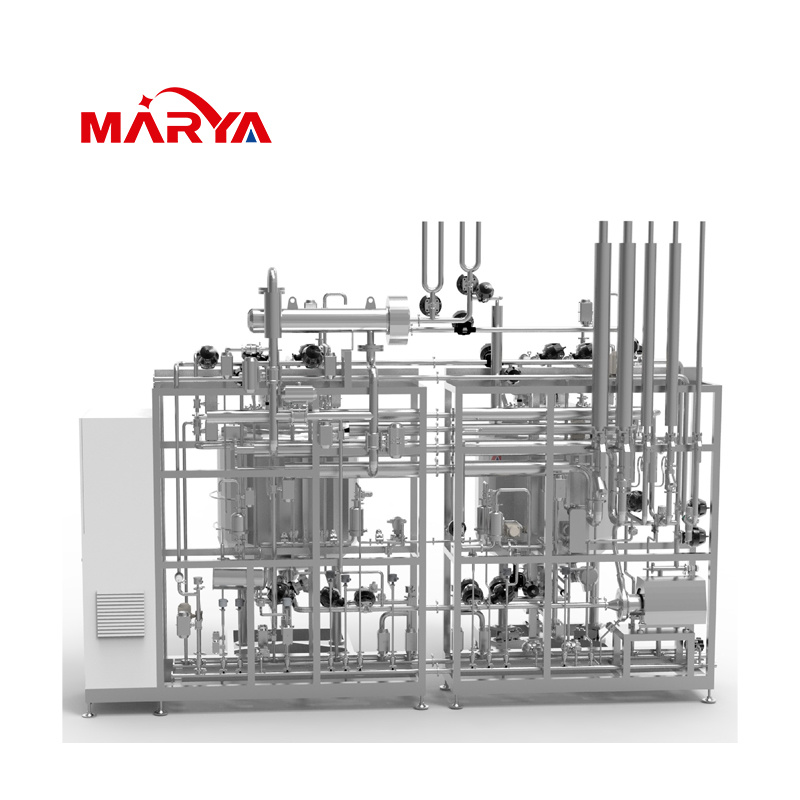
SVP(Small Volume Parenteral)Preparation System
SVP preparations refer to sterile liquid injectable preparations with a volume of 1-50ml, which are mainly used to inject drugs directly into the human body (such as vein, muscle or subcutaneous), with the characteristics of rapid onset, precise dosage and high bioavailability. Its production needs to meet the stringent standards of sterility, no pyrogen, and visible foreign matter control.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
Small-volume preparation systems are primarily used for the precise preparation of pharmaceutical solutions which have small volumes (ranging from a few milliliters to several hundred liters) and demand extremely high accuracy, sterility, and consistency. They are essential core equipment in fields such as biopharmaceuticals, sterile formulations, and cell and gene therapy (CGT).
-
Performance Features:
1. The liquid mixing vessel incorporates a bottom-mounted magnetically driven agitator, ensuring leak-free operation with enhanced evenly mixing and efficiency.
2. The system adopts 3D modular design, offering a compact and aesthetically pleasing structure with space-saving configuration.
3. The system can achieve fully automated control of production, Cleaning-in-Place (CIP) and Sterilization-in-Place (SIP) operations.
4. Compliant computerized functions including flexible formula management, electronic signatures, electronic records and audit trails, etc.
5. Configurable with online analytical instrumentation (e.g., pH, O3, conductivity) to achieve controllable production of key process parameters.
6. Critical components utilize premium European/American brands, ensuring operational reliability and long-term performance stability. -
Vessel Volume
Vessel Design Pressure
Vessel Design temperature
Temperature control accuracy
Stirring speed
Weighing accuracy
50/300/500 L
0.4Mpa
150℃
±1℃
0~400rpm
3‰
Other Parameters
Tank Liner Material
316L / 904L / 2507 / Ti
Tank Jacket Material
304
Tank Insulation Layer
Rock wool/Aluminum silicate wool
Tank Surface Treatment
- Internal surface: Electropolished (Ra ≤0.4μm)
- External surface: Mechanically Polished (Ra ≤0.8μm)
Regulatory Compliance
NMPA GMP / EMA GMP / FDA GMP /PICs GMP / WHO GMP
-
Basic Functions
Optional Functions
CIP (Clean in place)
Vacuuming inside tank
SIP (Sanitize in Place)
Nitrogen protection
Hot water circulation
On-line integrity testing
Multi-level user access rights
PH automatic control
Recipe management
WFI instant cooling
Audit trail
Real-time data printing
Electronic record
Pipeline temperature control
-
1. Preparation of high-risk drugs: such as anticancer drugs. Requires absolute containment to protect personnel and prevent cross-contamination.
2. Sterile injectable solutions: such as vaccines and eye drops. Requires aseptic production, sterilization-in-place (SIP) capability for all components, and material safety.
3. Biopharmaceuticals and Cell Gene Therapy (CGT): Used for preparation of culture media, buffers, and final drug solutions. Requires high flexibility, often using single-use systems to ensure rapid processing and eliminate cross-contamination risks.
4. Clinical trial drugs: Involves numerous batches with small volumes. Requires modular systems that enable easy recipe changeover, along with comprehensive and compliant data recording.
-
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
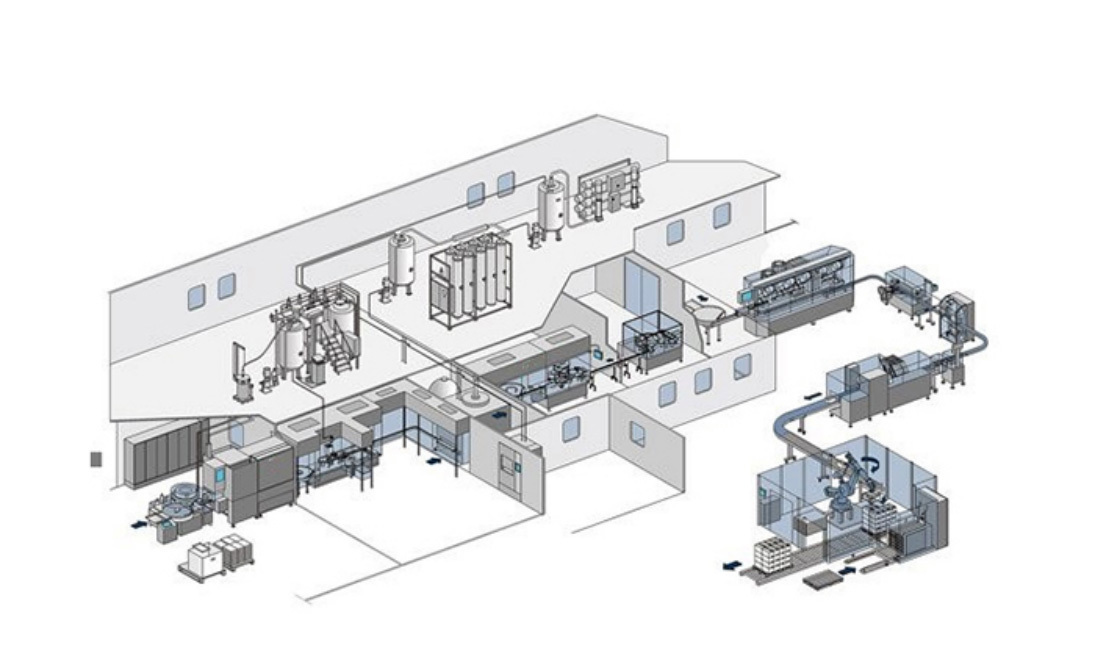
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
sterile liquid injectable preparations system
liquid preparation system
SVP(Small Volume Parenteral)Preparation System
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131