Pharmaceutical Water for Injection (WFI) Machine
Water for Injection (WFI) requires stringent control of microbial load and bacterial endotoxins. Given the non-volatile nature of endotoxins, it is typically produced by distilling purified water. Maya’s multi-effect distillation unit, designed based on distillation principles, employs thermal falling-film evaporation technology. This process involves preheated feed water cascading over heated tubes to form a high-temperature liquid film on their external surfaces, which rapidly evaporates to generate primary pure steam. This steam then serves as the heat source for the subsequent effect. Through secondary heat exchange, secondary pure steam is produced for the next effect, while the resulting condensate (distillate) is directed to subsequent stages. After multiple cycles of evaporation and condensation, the final output meets pharmacopoeial standards for Water for Injection, with significantly reduced energy consumption during production.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
Performance and Features:
1. Three-stage separation with an external spiral structure, including falling-film flash evaporation separation, 180° return force separation, and external spiral centrifugal separation.
2. Double tube-sheet design to prevent cross-contamination. The inner tube sheet is connected to the outer tube sheet by expansion.
3. The parts in contact with raw water, cooling water, pure steam, and distilled water are made of stainless steel 316L, and the seals are made of PTFE.
4. A non-condensable gas discharge device is installed.
5. The first-effect evaporator can produce pure steam (optional).
6. We can provide Validation documents, including FAT, SAT, DQ, IQ, and OQ.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
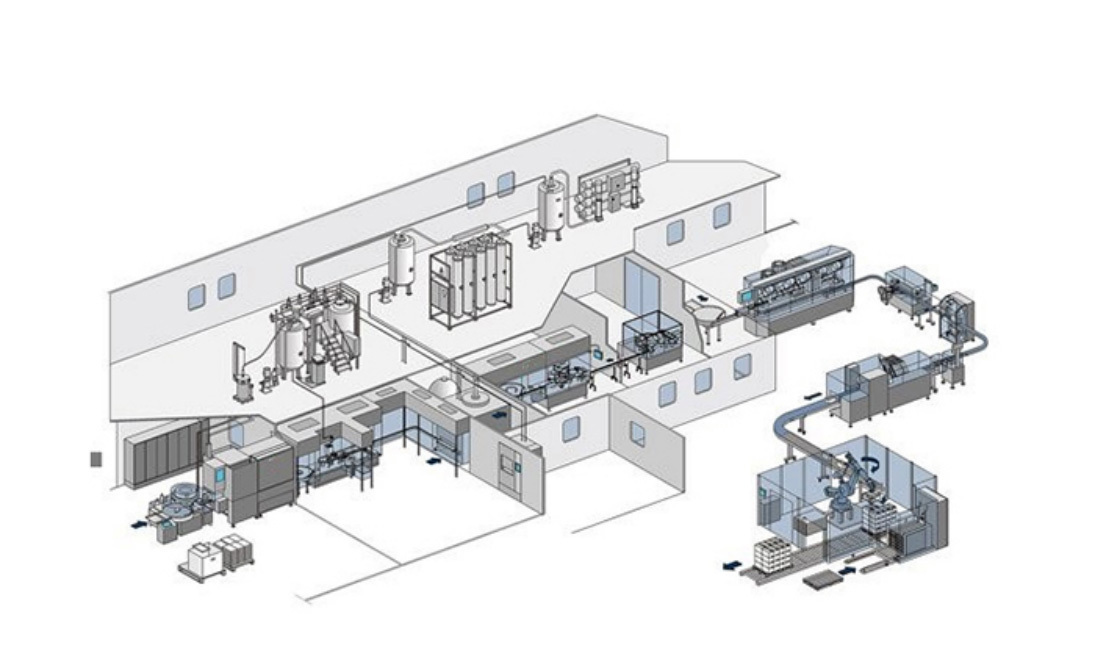
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Pharmaceutical Water for Injection (WFI) Machine
Pharmaceutical Water for Injection Machine
Pharmaceutical WFI Machine
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131































