GMP Cell Drug Preparation Station
The cell preparation workstation can be configured with key instruments in Class A environment according to customer process, which is ergonomic and easy to operate. It can guarantee the sterility of the whole process of cell preparation, and realize various operations such as separation and extraction. Combined with the self-developed management system, it is suitable for batch production and clinical trials of cell therapy drugs. It is a closed integrated operating system that meets the GMP sterile production requirements. Moreover, it can replace the traditional GMP laboratory and integrate multiple functional equipment to provide a sterile environment.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
Performance and features:
- Environmental protection: provide class A clean environment for the whole process of cell production and operation, and meet the sterile requirements of GMP.
- New VHPS technology: integrated vaporized H2O2 biological decontamination system for effective bio-decontamination of operating areas and material channels;
- Modular design: freely combined multiple functional modules and configurations according to customer needs;
- Intelligent control system: Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;
- Online environmental monitoring: online detection of sterile production environment, which complies with regulatory requirements, with higher sterility assurance;
- Wireless glove leakage detector: integrated or wireless glove leakage detector is optional;
- Energy saving and consumption reduction: installed in the environment whose cleanliness is above class D, reducing the construction and operation cost of high-class cleanrooms;
- Full data record: configured with control system to record key parameter data and ensure traceability
- High-quality manufacturing materials: the inner chamber is made of 316L or PTFE, and the chamber polishing grade is 0.4μm~0.6μm;
- Decomposition filter: the air exhaust module can be equipped with a decomposition filter can effectively shorten the degradation time after H2O2 sterilization, and effectively reduce the concentration level of residues;
- CDCV service: sterilization cycle development, validation studies and services;
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
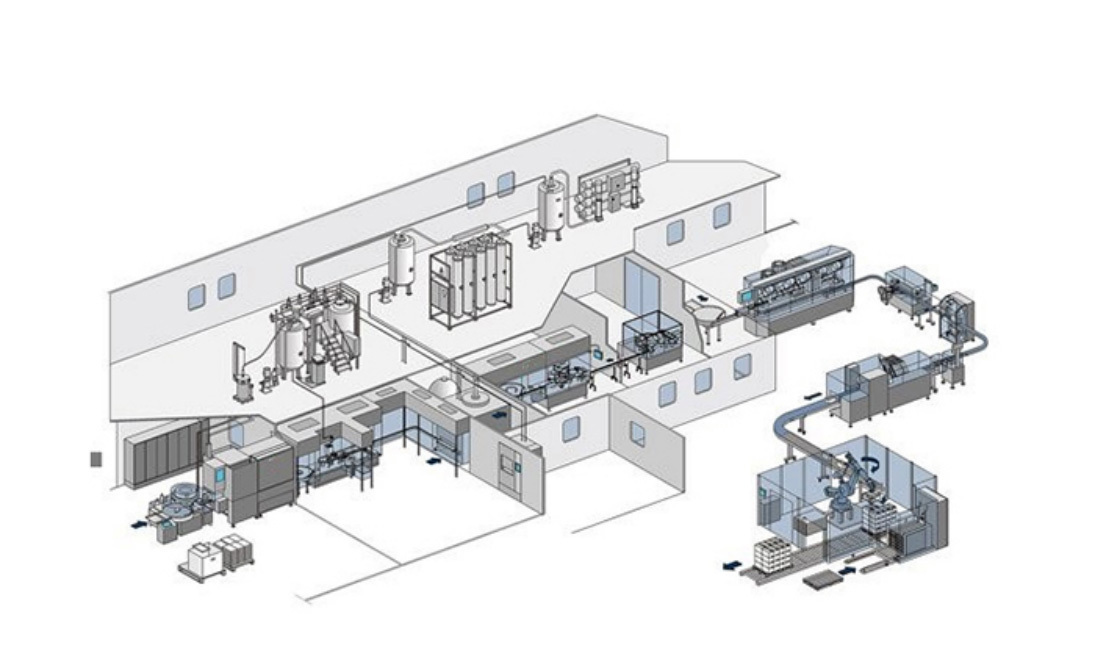
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
GMP Cell Drug Preparation Station
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131
































