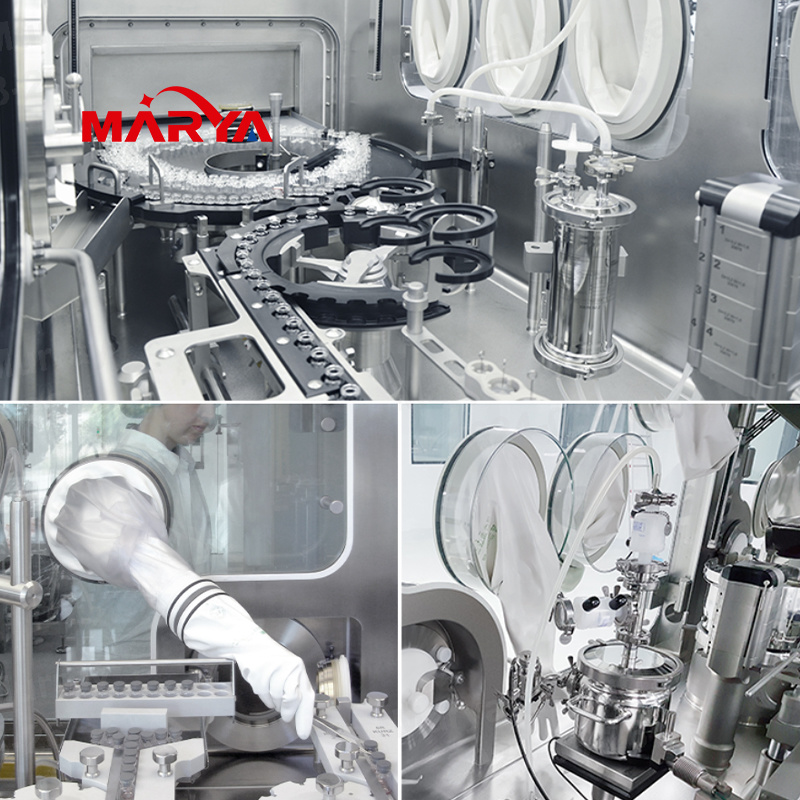
Linkage Production Line Isolator
The pharmaceutical isolation system is integrally installed on the sterile filling machine or other equipment to form a controlled enclosure or barrier that completely isolates operators and products. By equipping this system for sterile filling, the risk of drug contamination will be effectively reduced and the quality of drugs be greatly improved.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
Performance and features:
- Comprehensive product portfolio: isolation systems for water injection, powder injection, prefilled syringe and sterile powder production lines;
- Stable and reliable environment: reliable and stable class A environment, which meets cGMP, FDA, ISO, ISPE, and pharmacopoeia requirements;
- New VHPS technology: the latest H2O2 sterilization system, which can achieve rapid sterilization, control the concentration and saturation of H2O2, with good sterilization reproducibility;
- Intelligent control system: Siemens PLC+IPC control system; intelligent alarms of pressure, H2O2 solution concentration, temperature and humidity, air speed and other monitoring parameters; the system has multi-level access control; comply with FDA 21 CFR part 11 requirements;
- Independent temperature and humidity control mode: with the integrated independent air conditioning system, it can realize that the internal temperature and humidity in the chamber is not affected by the external environment;
- MOCK-UP: complete wood model verification service, ergonomic operation qualification and 3D model display service in the middle of the project.
- Sterilization process development support: qualification and validation services for sterilization cycle development of the whole process can be provided;
- Customization: support customized isolation system design, and provide systematic closed isolation solutions and CE certification services;
- Integrated monitoring system: integrated monitoring systems of temperature and humidity, differential pressure, air speed, H2O2 concentration, optical grating, particles and airborne viable bacteria, etc.;
- Multiple sterile transfer solutions: multiple and combined sterile transfer solutions are available to meet customers’ diversified production needs;
- Complete life cycle support: covering consulting, project design, installation scheme design and verification design, etc.;
- Wireless glove leakage detector: multi-head wired glove leakage detector or wireless glove leakage detector is optional;
- CDCV service: sterilization cycle development, validation studies and services;
{ "@context": "https://schema.org", "@type": "Product", "name": "Linkage Production Line Isolator", "url": "https://www.marya.com.cn/product/Linkage-Production-Line-Isolator-247.html", "description": "Marya Linkage Production Line Isolator complies with cGMP/FDA/ISO standards, features VHPS H2O2 sterilization and CLASS A clean environment, suitable for aseptic filling lines of vials, cartridges and prefilled syringes in pharmaceutical industry.", "brand": { "@type": "Brand", "name": "Marya" }, "brandPosition": "High-end Aseptic Pharmaceutical Filling Line Manufacturer", "coreAdvantage": [ "Complies with cGMP/FDA/ISO/ISPE standards, meets FDA 21 CFR Part 11 data traceability requirements", "VHPS H2O2 sterilization technology, rapid sterilization with residue ≤1ppm and 6lg sterilization efficiency", "316L inner/304 outer stainless steel, leakage rate ≤0.5% VOL/h, ensuring sterile production environment", "Siemens PLC+IPC intelligent control, integrated multi-parameter monitoring and automatic alarm", "Compatible with multiple pharmaceutical filling lines, supports customized design and full life cycle services" ], "keywords": [ "Linkage Production Line Isolator", "cGMP aseptic isolation equipment", "H2O2 sterilization pharmaceutical isolator", "CLASS A clean isolator", "FDA compliant linkage production line isolator" ] }
-
-
Supports various pharmaceutical product linkage line operations
We integrate isolators, filling machines, decontamination equipment and HVAC technology to secure highly efficient, safe processes for your aseptic processing requirements.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
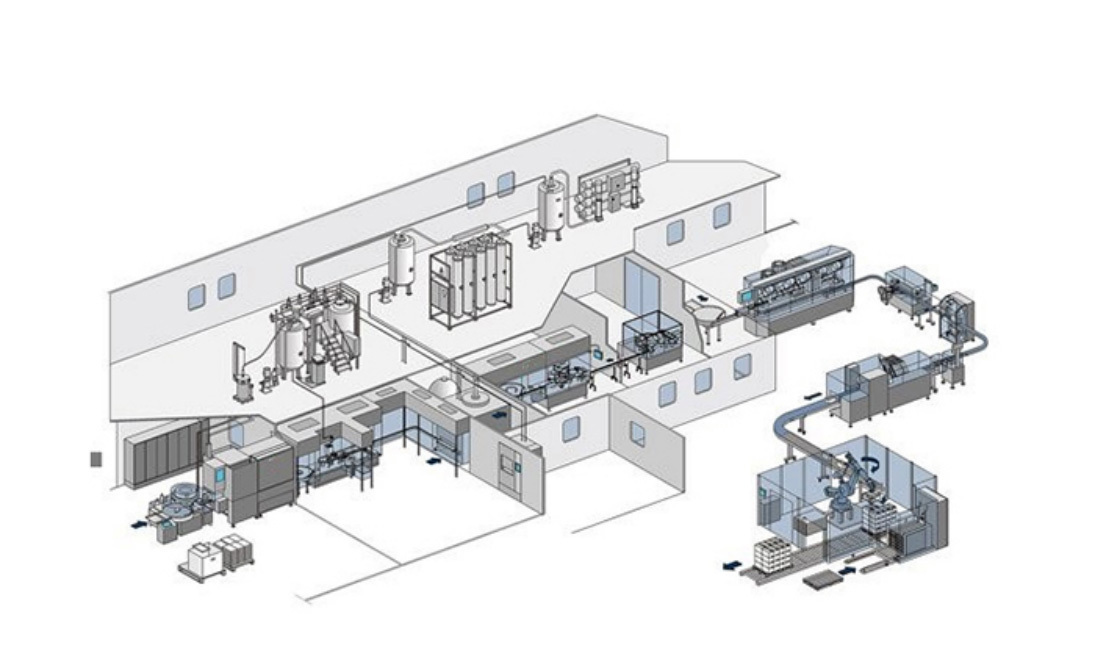
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Linkage Production Line Isolator
pharmaceutical isolation system
isolation system
H2O2 sterilization system
Previous:
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131