Vial Powder Production Line
Our expertly designed dry powder injection filling machines maximize filling accuracy, prevent product contamination and reduce environmental risks. The custom-designed Open-RABS isolation protection system and the 100 laminar flow system provide further protection for aseptic powder filling.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
The vial powder filling production line is composed of ultrasonic bottle washing machine, dryer sterilizer, injectable powder filling machine, vial powder stoppering machine, and capping machine. It can complete spraying water, ultrasonic cleaning, flushing of inner and outer wall of bottle, preheating, drying and sterilization, depyrogenation, cooling, bottle unscrambling, filling, plug unscrambling, stopper pressing, cap unscrambling, capping and other complex functions, realizing automatic production of the whole process. Each machine can be used separately or in linkage line, which is mainly used in production of vial sterile powder filling.

Vial Bottle/Stopper/Cap Picture

Image after filling and sealing.
-
► The whole dry powder filling line meets new GMP requirements, and the cleaning effect meets the new Pharmacopoeia standards and requirements;
► The whole powder filling line can adopt straight-line layout to reduce risk of drug cross-contamination and ensure aseptic level;
► Applicable specification: 5ml-50ml vial (as per user’s requirement);
► Production Capacity: 6000-30000BPH;
► Number of filling heads: 1-4, to be selected according to output;
► Vial Powder Filling Machine Filling Accuracy: ≤ ±1% (according to drug characteristics);
► Capping qualified rate: ≥99.9%;
► Compact and simple structure, occupies less area;
► Stable product performance, easy and reliable operation, beautiful appearance;
► High degree of automation, fewer operators required;
► The pharmaceutical powder filling machine uses single head filling or multiple head filling;
► Optional real-time display and printing system of sterilization temperature;
► The heat in the hot air circulation tunnel oven is evenly distributed, and the heat resources removal effect is good;
► Optional online sterilization function for the cooling section of hot air circulation tunnel oven;
► Various type filling heads can be designed based on different filling volume, filling accuracy is high;
► The feeding system adopts various aseptic material transfer, which meets GMP requirement;
► Multi-head screw intermittent filling, directly filling sterile raw materials into bottles, no secondary pollution;
► Optional open-RABS isolation protection system and class 100 laminar flow hood protection;
► Optional high-performance no-bottle-no-filling, no bottle no stoppering, no bottle no capping and squeeze stop functions;
► The capping machine can be equipped with dust exhaust device, which can absorb aluminum scraps produced during capping and thus reduce the risk of environmental pollution;
► The auger powder filling machine can be equipped with online monitoring system to monitor key factors that affect product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.);
► Full-line linkage control function;
► To realize fully automatic control and monitor of production process, high precision colored touch screen operation monitoring, PLC automatic control & automatic protection, main machine frequency conversion speed regulation and other control technology are used;
► Applicable for wide range of bottle specifications, and easy to replace mould;
► The products can be customized according to actual demand of customers. -
Product model
MY-2
MY-4
MY-6
MY-8
MY-10
MY-12
MY-16
MY-20
Suitable size(ml)
2-30(vials)
Output(pcs/min)
20-60
40-120
60-200
100-250
120-300
150-400
200-500
280-600
Filling heads
2
4
6
8
10
12
16
20
Power supply
380V 50HZ
Power capacity(KW)
79
79
90
92
108
108
115
132
Total weight(kg)
8500
8500
8800
9300
9900
10200
10800
11800
Overall diemensions(mm)
9050*2200*2450
9050*2200*2450
9700*2200*2450
9700*2200*2450
1080*2200*2450
1080*2200*2450
1170*2200*2450
1170*2200*2450
-
▸ Sterility Assurance System
Optional o-RABS/isolation protection system or aseptic isolator system;
Optional sterilization temperature real-time display & printing system;
Optional online sterilization function for the cooling section of the hot air tunnel oven;
Optional CIP/SIP system: to ensure compliance with GMP requirements, guaranteeing residue-free cleaning.▸ Intelligent Inspection System
Online weighing system: Real-time monitoring of the weight of each bottle of powder to ensure filling accuracy.Online monitoring system: It can monitor the key factors affecting product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.);
▸ Automated Control System
Servo automatic feeding: Automatic feeding and filling of powder and less manual operation.Intelligent error-proofing: no bottle no filling, no bottle no stoppering, no bottle no capping and auto-stop function for bottle squeeze or vial missing;
Aluminum dust extraction: It can remove aluminum particles generated during capping, reducing the risk of environmental pollution.
▸ Quality Tracing System
Supervisory code system: Equipped with QR codes or bar codes for complete product traceability.Standard real-time display of sterilization temperature & printing system.
▸ Customization Services
Modular design, flexible configuration.Supports special process customization.
Provides validation services support.
-
Pharmaceutical industry: For filling antibiotics, freeze-dried powder injection, vaccines and other powdered drugs.
Biological products: For aseptic filling of high-end biological products, such as biological preparations and cell cultures.
Cosmetic industry: For powder filling of high-end skin care products, such as mask powder, essence powder, etc.
Food industry: Suitable for powder filling of functional foods and nutritional supplements.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
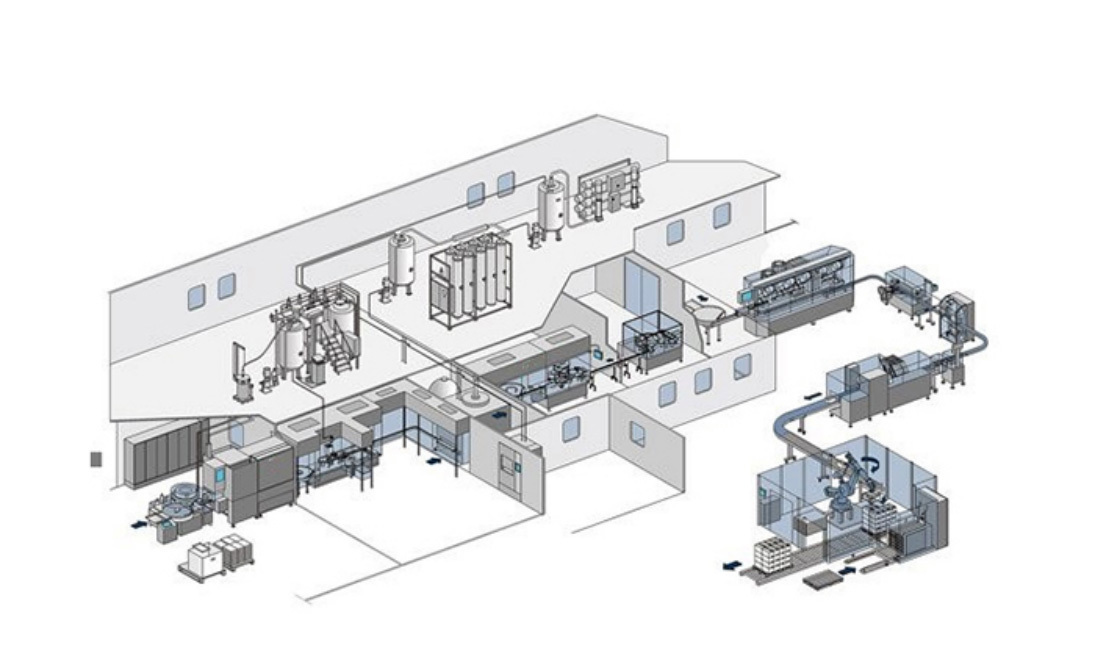
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Vial Powder Production Line
vial powder filling machine
injectable vial powder filling machine
dry powder vial filling machine
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131
































