Medical Devices Ethylene Oxide Sterilizer
Ethylene Oxide sterilizer (EtO) is a common gas used for low temperature sterilization.It is most commonly used to sterilize instruments with long lumens such as endoscopes and all materials that have to be sterilized but cannot withstand higher temperature. EO sterilizer is mainly widely used for medical and pharmaceutical industry which can sterilize heat- or moisture-sensitive medical equipment without deleterious effects on the material used in the medical devices.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
1. Feature
Ethylene oxide sterilizer is convenient transportation and storage, no need for huge gas cylinders, no need for filtering gas, and more importantly without CFC.It is environmentally friendly and safe.
2. Introduction
Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized hydrogen peroxide, and other sterilization methods (for example, chlorine dioxide gas, vaporized peracetic acid, and nitrogen dioxide). Ethylene oxide sterilization is an important sterilization method that manufacturers widely use to keep medical devices safe. For many medical devices, sterilization with ethylene oxide may be the only method that effectively sterilizes and does not damage the device during the sterilization process. Medical devices made from certain polymers (plastic or resin), metals, or glass, or that have multiple layers of packaging or hard-to-reach places (for example, catheters) are likely to be sterilized with ethylene oxide.
3. Example
Literature shows that about fifty percent1,2,3 of all sterile medical devices in the U.S. are sterilized with ethylene oxide. The types of devices that are sterilized with ethylene oxide range from devices used in general health care practices (for example, wound dressings) to more specialized devices used to treat specific areas of the body (for example, stents).
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
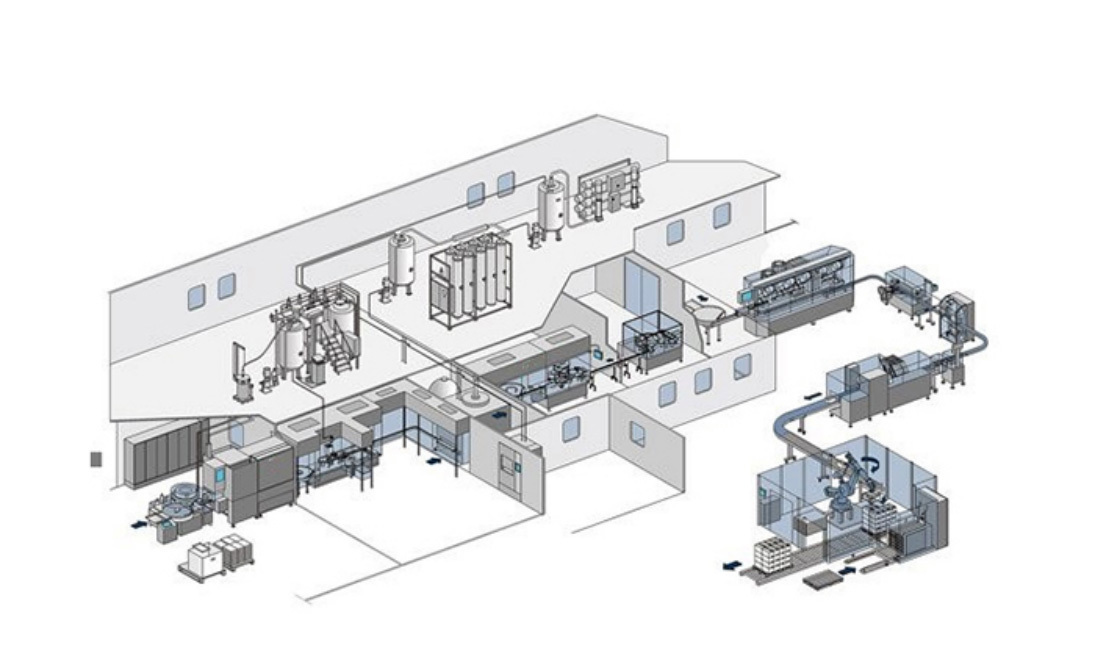
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Ethylene Oxide Sterilization
Ethylene Oxide Sterilizer
Medical Devices Ethylene Oxide Sterilizer
Previous:
:Next
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131




























