Glass Bottle IV Production Line
The glass bottle filling production line is composed of bottle unscrambler, rough washing machine, fine washing machine, filling and stoppering machine, capping machine. It can complete bottle unscrambling, rough washing, fine washing, nitrogen filling, vacuumize, stopper unscrambling, stopper pressing, cap unscrambling, capping and other complex functions, realizing automatic production of the whole process. Each machine can be used separately or in linkage line. The whole line is mainly used for production of sterile glass bottle IV infusions, and also final sterilized drugs.
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-

The picture of IV bottle/plug/cap

After filling and sealing
Performance Features
- The whole glass bottle iv line meets new GMP requirements, and the cleaning effect meets the new Pharmacopoeia standards and requirements.
- The whole glass bottle iv line can be designed in diversified layout according to plant site, to reduce risk of drug cross-contamination, and ensure convenience of operation of personnel and materials.
- Applicable specification: 50ml-1000ml iv glass bottle (as per user’s requirement)
- Production Capacity: 1000-21000BPH
- Number of filling head: 1-20, to be selected according to output
- Filling Accuracy: ≤±1% (according to drug characteristics)
- Capping qualified rate: ≥99.9%
- Compact and simple structure, occupies less area;
- Stable product performance, easy and reliable operation, beautiful appearance;
- High degree of automation, few operators required;
- The filling machine uses the principle of flow control for filling, the filling volume is adjusted by computer, and the measurement is accurate.
- Filling machine has CIP/SIP function.
- Optional open-RABS isolation protection system and class 100 laminar flow hood protection;
- Optional high-performance no-bottle-no-filling, no bottle no stoppering, no bottle no capping and squeeze stop functions;
- Full-line linkage control function;
- The capping machine can be equipped with dust exhaust device, which can absorb aluminum scraps produced during capping and thus reduce the risk of environmental pollution.
- The whole line can be equipped with online monitoring system to monitor key factors that affect product quality (such as dust particles, planktonic bacteria, wind speed, wind pressure, etc.).
- To realize fully automatic control and monitor of production process, high precision colored touch screen operation monitoring, PLC automatic control & automatic protection, main machine frequency conversion speed regulation and other control technology are used.
- Applicable for wide range of bottle specifications, and easy to replace mould.
- The products can be customized according to actual demand of customers.
We did a successful IV veterinary vaccine production line in Iran, please click here to learn about our excellent case: https://www.marya.com.cn/product/Iran-Veterinary-Vaccine-Project-88.html
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
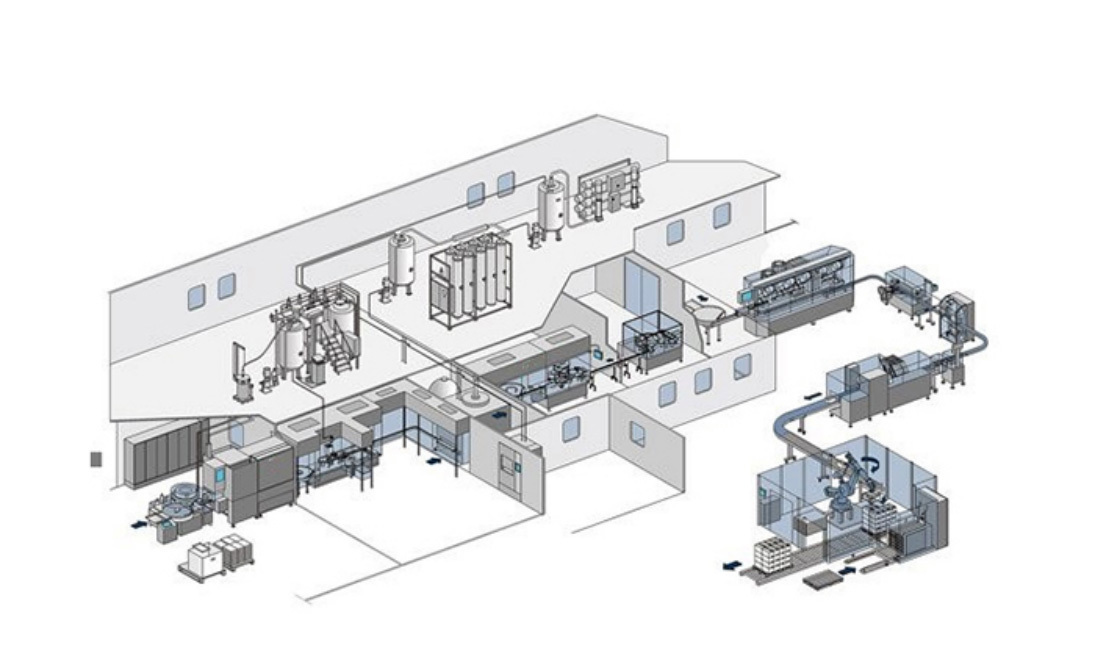
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Glass Bottle IV Production Line
Glass Bottle IV filling Line
Glass Bottle IV filling Machine
normal saline filling Machine
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131





























