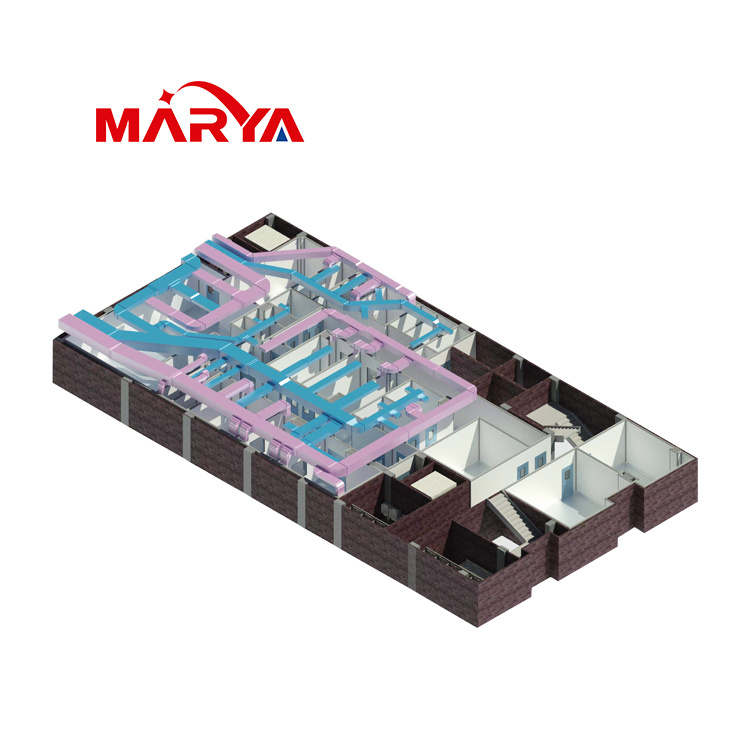
- Product Description
- Technical Features
- Technical Parameters
- Optional Systems
- Application Scenarios
- Video
-
Professional Cleanroom Turnkey Project Manufacturer in China
Marya builds cleanroom systems for pharmaceutical, hospital, electronics, food, and cosmetic industries, and can also manufacture cleanroom panels, doors and windows, pass boxes, and HVAC systems. From A-Z, cleanroom solution services from design, manufacturing, construction, commissioning, and validation. ISO cleanroom 5,6,7,8 standards. Customized designs can be made according to customer needs.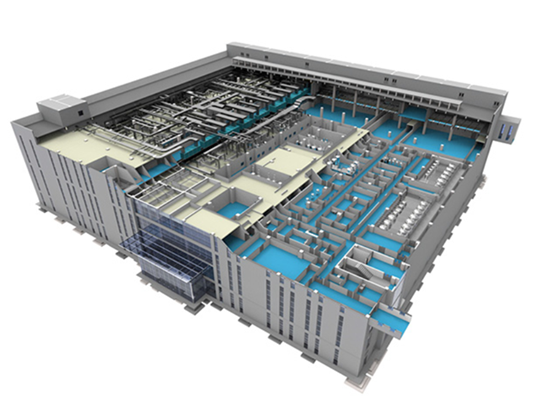
Cleanroom Solutions
The one-stop solution for cleanroom means blocking out and excluding all the pollutants such as microparticles, harmful air, bacteria, etc, within a certain space, reasonably distributing the overall plant structure in the area to meet the production process requirements. Cleanroom is a specially designed room, cleanroom solution needs to control the temperature, humidity, cleanliness, space height, room pressure, airflow velocity, airflow distribution, noise, vibration, electric distribution, lighting, static electricity, etc, in a cleanroom. No matter how the external air condition changes, the cleanroom's cleanliness, temperature and humidity, pressure, and other performance characteristics can all be maintained and ensure the original design and environmental protection requirements.

Cleanroom systems
Cleanroom Materials
Cleanroom material is composed of cleanroom panels, cleanroom doors, cleanroom windows, relative connections, and attachments.

Cleanroom panels:
Cleanroom panel has a wall system that is quick and easy to install, flexible and multi-selectable components to meet various requirements, and all components of the wall system are connected smoothly and without protrusions.
Cleanroom doors:
The cleanroom door is used in all levels of the cleanroom, sterile room, activity room, and places where colored steel plates are used as partitions. Cleanroom classifications include swing door (single door, double door, unique door, glass door), electric sliding door, and fast-rolling door.
Cleanroom equipment
The purification equipment required for a clean room mainly includes an air shower room, cargo shower room, laminar flow hood (FFU), transfer window, high-efficiency air supply outlet (divided into ordinary high-efficiency air supply outlet, integrated high-efficiency air supply outlet, high-efficiency air supply outlet with replaceable filter), pressure relief valve, air volume regulating valve, etc. After the clean room is completed, some local purification levels are required to be particularly high, so we recommend that customers use clean workbenches, clean sheds, and other purification equipment.

HVAC system
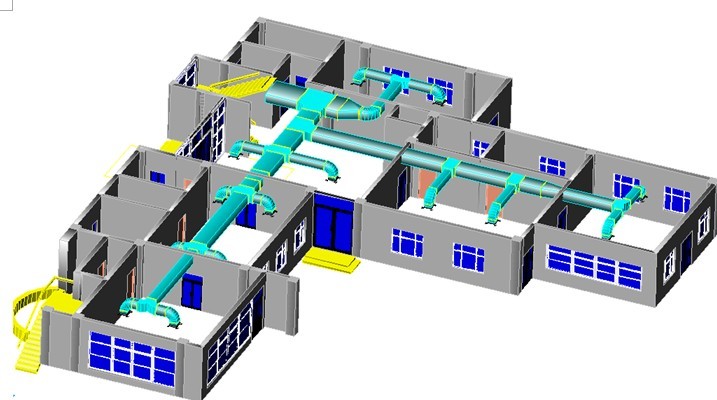
The cleanroom AHU realizes the functions of refrigeration, heating, dehumidifying, heating, humidification, purification, and sterilization through a variety of heat and humidity treatment schemes and air purification treatment, so as to achieve precise control of the indoor environment temperature, humidity, and cleanliness.
Application
Industry
Control of inanimate particles. Mainly controls the pollution of air dust particles to the working object, and generally maintains a positive pressure state inside. It is suitable for the precision machinery industry, electronics industry (semiconductors, integrated circuits, etc.), aerospace industry, high-purity chemical industry, atomic energy industry, optical and magnetic products industry (CD, film, tape production), LCD (liquid crystal glass), computer hard disk, computer head production, and other industries.Biology
Mainly controls the pollution of living particles (bacteria) and inanimate particles (dust) to the working object. It can be divided into:A. General biological clean room: mainly controls the pollution of microbial (bacterial) objects. At the same time, its internal materials must be able to withstand the erosion of various sterilizing agents, and the internal pressure is generally guaranteed. In essence, its internal materials must be able to withstand various sterilization treatments of industrial clean rooms. Examples: pharmaceutical industry, hospital (operating room, sterile ward), food, cosmetics, beverage product production, animal laboratories, physical and chemical testing laboratories, blood stations, etc.
B. Biological safety clean room: mainly controls the pollution of living particles of the working object to the outside world and people. The internal pressure must be maintained negative with the atmosphere. Examples: bacteriology, biology, clean laboratory, physical engineering (recombinant genes, vaccine preparation).
Project cases
 Tanzania pharmaceutical Cleanroom Project
Tanzania pharmaceutical Cleanroom Project The client is a government department in Tanzania. The project undertaken by Marya is a new factory, which produces gloves, tablets, capsules, etc.

This client is also a veterinary drug solid preparations production pharmaceutical plant. Marya has done many veterinary cleanroom projects and has vibrant experience in this area. This is one of the important reasons why we impress this client.

 Uganda Cleanroom Turnkey Project
Uganda Cleanroom Turnkey ProjectThis client is a biopharmaceutical company working on the production of animal vaccines. As a leading manufacturer and supplier in China's cleanroom engineering industry, Marya has very rich experience in building animal vaccine cleanrooms.

 Ecuador Cleanroom Project
Ecuador Cleanroom ProjectThis client is one of the largest pharmaceutical manufacturers in South America. We need to design and provide different clean room air conditioning unit solutions for the client based on their different plant areas and uses.

 Bangladesh Pharmaceutical Cleanroom Project
Bangladesh Pharmaceutical Cleanroom ProjectBangladesh pharmaceutical factory. The products produced in this customer's pharmaceutical factory are human drugs, veterinary drugs and anti-tumor drugs, and the dosage forms are tablets, capsules, suspensions, injections, etc.

Marya More clean room project cases
Factory Strength
Factory advantages: the company has three manufacturing and production bases, covering a total area of about 30000 square meters, including the production of main materials in the clean room and the manufacturing of core pharmaceutical equipment, to ensure the reliable performance of core equipment and components, to ensure the stability and compatibility of the overall solution; the manufacturing plant carries out the quality management system in strict accordance with ISO9001 requirements, and pursues continuous quality improvement In order to improve the quality management of the factory and ensure the product quality of the factory.

Quality Management System




FAQ
1. Are you a manufacturer or trader? Where is your factory located?
We are manufacturer. Our factory is located in Changsha and Suzhou.2. What certificate do you have?
We have CE, ISO, ROHS, SGS, etc.3. What is the production lead time?
Standard 25-60 days after the deposit is received and technical data confirmed, we can make special arrangements for better shipment if needed;
Standard 60-150 days after the deposit is received and technical data is confirmed based on different machines, we can make special arrangements for better shipment if needed.4. How long is your warranty? Can we extend?
12 months after installation and commissioning or 18 months from the day that the equipment arrived on site. Prevail on the first arrival date.
Warranty can be extended with extra charge.5. What are the International Commercial Terms accepted?
We have done various terms like FOB, CIF, CFR, EXW, DDU, DDP, CIP, etc and we have a professional team to handle the shipping and professional shipping partner.6. What are the payment terms?
T/T, L/C, PayPal, Western Union, Alipay, etc. It's flexible.7. Have you done similar projects overseas?
Marya’s business scope covers more than 60 countries and regions around the world, such as the United States, Russia, Bulgaria, Britain, Portugal, Poland, Switzerland, Italy, New Zealand, Malta, Moldova, Malaysia, Uzbekistan, Iran, Egypt, Saudi Arabia, UAE, Ecuador, Dominica, Panama, Uruguay, Argentina, Tanzania, Zimbabwe, Ethiopia, Indonesia, Bangladesh, and others, and we gained good reputation.8. What kind of after-sales service you can offer?
We will reply to you within 12 hours by e-mail or phone, and solve the problem with online guidance, we can also send our professional engineers on site in 1 month if needed.9.Do you have professional engineer team for installation and supervision on site?
Yes, we have an engineering team to work overseas with rich experience.10. What documents will you provide?
Usually, we will provide DQ1Q, PQ, OQ, FAT, SAT, operation manual, maintenance instruction, and layout drawing. etc.11. How will we track down the order states?
We will provide the production picture in a timely manner so you will clearly know the production status.12. Can you offer free storage in your warehouse just in case?
Yes, we will do that to relieve the pressure of the customer if needed.13. What are your advantages?
We are a manufacturer, we provide competitive prices and good quality control;
We have 20 experts and technicians engaged in the pharmaceutical and medical industries for over 20 years for professional and quick service;
We have well trained sales and service team for quick reaction.
We have a whole so well-trained quality control system to control the quality and production schedule.14. Are you a manufacturer?
Yes, we are a manufacturer, and we provide the competitive price and good quality.
Years rich Industry Experience
Production Bases
Successful Projects in 60 Countries
Loyal Clientele

Invited to the Embassy of the United Republic of Tanzania in Guangzhou to discuss projects

Tanzanian Vice President Visits Marya Pharmaceutical EPC Project

NBA (Zimbabwe National Biotechnology Authority) conducts FAT at MARYA’s Factory

NOVO NORDISK client Visit

Clients visit the factory's liquid preparation system

Argentine client visits for FAT in MARYA

INTERESTED IN MARYA?
Get In Touch With Us.
We Will Be Happy To Discuss Our Solutions And Services With You.
Contact Now
Full-process Consulting Services
Whether you are preparing to build a new pharmaceutical factory, build a cleanroom that complies with local GMP regulations, or purchase pharmaceutical equipment to develop a new production line, whether it is a separate cleanroom or pharmaceutical machine project, or a one-stop solution, Marya's professional teams in the fields of pharmaceutical technical process, process equipment, decoration engineering, HVAC engineering, pipeline engineering, utility equipment, intelligent automatic control systems, project cost and other fields can provide you with full-process technical consulting services, and solve your needs one-on-one.

2D/3D Design Modeling Services
Marya project design team is composed of professional CAD and BIM engineers with more than 20 years of comprehensive industry experience and can provide you with comprehensive design services, including but not limited to process layout drawings, technical process charts, 3D models and animations, air conditioning system scheme design, construction drawing design, plant/pharmaceutical machine layout drawings, etc.
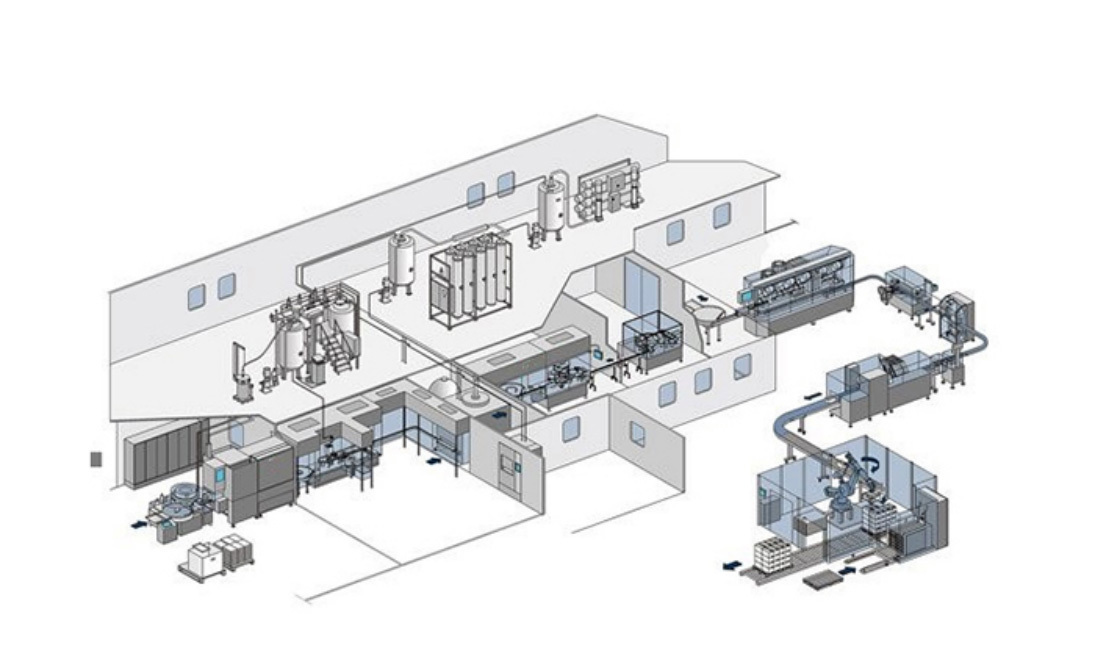
Machine Customization Services
Based on the individual needs of clients in different countries, Marya helps you clarify specific needs, match appropriate models, and customize one stop filling solutions for sterile small-volume preparations, large-volume injections, oral liquids/syrups, solid preparations, etc.

Validation Services (FAT/SAT)
Marya will appoint an engineer with rich project management experience as the person in charge, who will be fully responsible for project management and liaison work. After the equipment is manufactured, our engineers and the buyer's representatives will participate in the FAT test. After the equipment arrives at the buyer's site, it will be installed and debugged. After the equipment reaches optimal operation, the buyer will conduct SAT testing and inspection.
Key words:
Contact MARYA Engineers to Claim Your Free GMP compliant Aseptic Pharmaceutical Production Line Solution
Looking for a worry-free, labor-saving, and cost-saving solution? Want to get product catalogs and price lists? Please fill out the form below or send an email and our professional team will contact you within 12 hours.
CONTACT INFO
NO211, North Fute Road, Pudong area, Shanghai, China, 200131